-40%
Digital thermometry Medical infrared thermometer Non-Contact Body Temperature,CE
$ 9.49
- Description
- Size Guide
Description
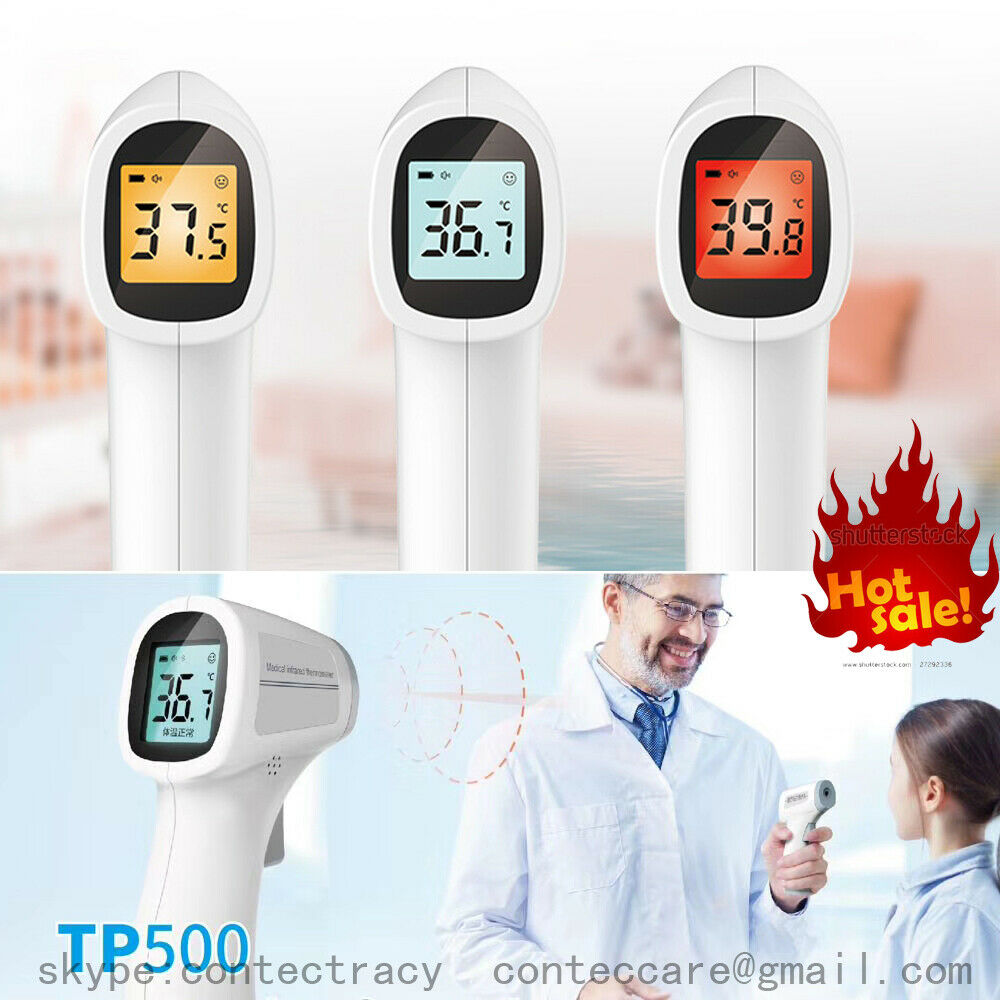
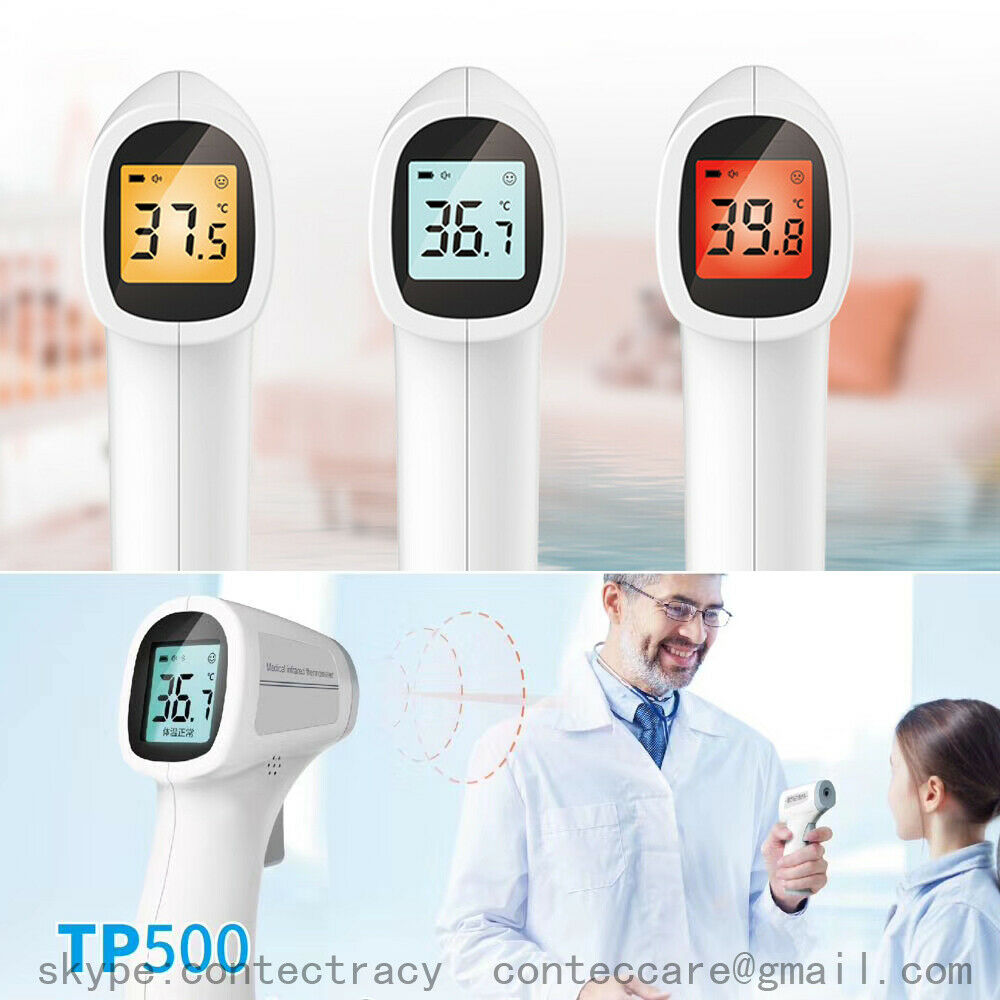
Medical infrared thermometer TP500Product name: Medical infrared thermometer
Safety type: Internal power supply BF type pplication part.
Protection against liquid penetration: IPXO
Safety lassification: equipment that cannot be used in thepresence of a mixture of flammable anesthetic gas and air orwith oxygen or nitrous oxide
. Operation mode: continuous operation
. Temperature unit: Celsius (℃)/ Fahrenheit(*F)
. Resolution: 0.1℃
. Temperature display range:32℃ ~ 42.9℃
. Maximum allowable error:
Within the range of 35.0℃ ~ 42.0℃
the maximum allowableerror is + 0.2℃ Within the range of less than 35.0℃and more than 42.0℃, the maximum allowable error is 0.3℃
. Maximum allowable clinical repeatability:
less thant 0.3℃
.
Measurement time: less than 1 second
.
Display mode: LCD display
.
Memory data: 30 groups
. Power management: intelligent shutdown, ultra-low power,
consumption control; power display; low power reminder
. Power supply: DC3 V(2 AAA batteries)
. Duration ofuse: 5 years
Body weight: about 130g (TP500)
. Normal working and storage conditions:
Temperature range:
Normal work: 16℃~ 35 ℃;
Transportation and storage: -20℃~+55 ℃;
Humidity range:
Normal operation: <85% (non-condensing);
Transportation and storage: <95% (non-condensing);
Atmospheric pressure:
Normal operation: 700 hPa ~ 1060 hPa;
Transportation and storage: 500 hPa ~ 1060 hPa;
Shipping
By Airmail: Generally, about 2-3 weeks
By Express: Generally, about 5-10 working days to UK, USA, AU and so on.
We can also ship the item through express service like DHL, UPS, if you are ready to pay the extra charge.
Terms of Sale
Buy Safe Product!
The following FDA Disclaimer is required for all eBay listing in Healthcare category and is included for REFERENCE:
The sale of this item may be subject to regulation by the U.S. Food and Drug Administration and state and local regulatory agencies. If the item is subject to FDA regulation, We will verify your status as an authorized purchaser of this item before shipping of the item.
The Fingertip Pulse
Oximeter is registered on the Australian Register of Therapeutic Goods (
ARTG
)
with the code
197923
,
and certified by
FDA
of United States and
CE
,
TUV
of Europe.
The Fingertip Pulse Oximeter that is
FDA 510K
Approved
Taxes, Customs & Duties
There are not extra taxes, VAT or other unmentioned payment. You pay us with goods subtotal + shipping cost (not include duties). But you need to pay for the import duties in case that occurred for certain goods.
Return - After service
We offer 60 days return policy. 100% satisfaction is our goal!
1.All items are brand new ,with 1 years warranty.
2.We will refund the money to you when we get the return items or replace item for you.
About Us
Contec Medical Systems focusing on research, manufacture and distribution of medical instruments,was founded in 1992 as a high-tech company.At present there are more than 1200 employees in our company.
We are looking forward to establishing a successful business relationship with you.
Only english user guide,if you need any other language,please contact me.